Answer: Reduction
Explanation: Reduction is a chemical reaction in which electrons are gained and thus there is a decrease in the oxidation number.
Oxidation is a chemical reaction in which electrons are lost and thus there is a increase in the oxidation number.
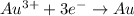
This is an example of reduction reaction as the oxidation number of gold has decreased from +3 to zero as the electrons are gained.