Step 1
Information provided:
125.0 mL of 0.100 M HNO3
80.0 mL of 0.0750 M Ca(OH)2
-------------
Step 2
Molarity is defined as follows
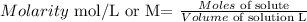
------------
Step 3
The number of moles in addition from each solution:
HNO3)
125.0 mL = 0.125 L
(1 L = 1000 mL)
Moles HNO3 = Molarity x Volume (L)
Moles HNO3 = 0.100 M x 0.125 L = 0.0125 moles
--
Ca(OH))
80.0 mL = 0.080 L
Moles = 0.0750 M x 0.080 L = 6x10^-3 moles
------------
Step 4
HNO3 and Ca(OH)2 are strong compounds, so:
HNO3 => H+ + NO3-
0.0125 moles 0.0125 moles 0.0125 moles
---
Ca(OH)2 => Ca2+ + 2 OH-
6x10^-3 moles 6x10^-3 moles 2 x 6x10^-3 moles = 0.012 moles
Number of moles of H+ = 0.0125 moles
Number of moles of OH- = 0.012 moles
It appears to be neutral but according to our calculation: The number of moles of H+ = 0.0125 moles > Number of moles of OH- = 0.012 moles
So, the mixture is acidic
Answer: acidic