Step-by-step explanation:
A chemical reaction equation that contains same number of atoms on both reactant and product side is known as a balanced equation.
For example,
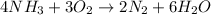
Here, number of nitrogen atoms on both reactant and product side is 4.
Number of hydrogen atoms on both reactant and product side is 12.
Number of oxygen atoms on both reactant and product side is 6.
Since, there are 4 moles
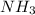 and 2 moles of
and 2 moles of
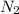 .
.
Therefore, we can conclude that mole ration of
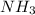 to
to
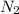 is 4:2 which is equal to 2:1.
is 4:2 which is equal to 2:1.