Answer: This compound is made up of 1 mole of ferric ion and 3 moles of chloride ions.
Step-by-step explanation:
The given compound is an ionic compound and is made up of ions: a cation and anion.
The name of
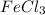 is ferric (III) chloride.
is ferric (III) chloride.
This compound contains 1 mole of Ferric ions
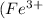 and 3 moles of chloride ions
and 3 moles of chloride ions
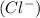
Ionization of
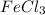 follows the equation:
follows the equation:
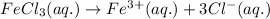
Hence, this compound is made up of total 4 ions.