Answer: Option (c) is the correct answer.
Step-by-step explanation:
Molecular formula of water is
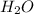 . As oxygen is more electronegative than hydrogen atom hence, it will attract the electrons more towards itself.
. As oxygen is more electronegative than hydrogen atom hence, it will attract the electrons more towards itself.
As a result, a partial positive charge will develop on hydrogen atom and a partial negative charge will develop on oxygen atom.
Hence, water molecule become polar in nature.
Thus, we can conclude that water considered a polar molecule because the atoms in the molecule have partial charges.