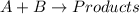Answer : The correct option is, (C)
Explanation :
Chemical equation : It is written in such a way that the symbolic representation of the reaction represents the reaction taking place in the system.
The reactants are written on the left-hand side and the products are written on the right-hand side of the equation and the reactant and products are separated by an arrow. The two or more reactants and products are separated by “+”.
Hence, the representation of chemical reaction will be,