Answer: The reducing agent of the given chemical reaction is
Step-by-step explanation:
Reducing agent is defined as the agent which reduces the other substance and itself gets oxidized. It undergoes oxidation reaction. Oxidation reaction is defined as the reaction in which a substance looses its electron. The oxidation state of the substance gets increased.
Oxidizing agent is defined as the agent which oxidizes the other substance and itself gets reduced. It undergoes reduction reaction. Reduction reaction is defined as the reaction in which a substance gains electron. The oxidation state of the substance gets reduced.
For the given chemical reaction:
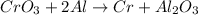
On reactant side:
Oxidation state of chromium = +5
Oxidation state of aluminium = 0
Oxidation state of oxygen = -2
On product side:
Oxidation state of chromium = 0
Oxidation state of aluminium = +3
Oxidation state of oxygen = -2
As, oxidation state of aluminium is getting increased from 0 to +3. Thus, it is getting oxidized and considered as reducing agent. The oxidation state of chromium is getting reduced from +5 to 0. Thus, it is getting reduced and is considered as oxidizing agent.
Hence, the correct answer is aluminium.