Calculate the mass, in amu, of 278 atoms of Li.
1) We need to find Li (Lithium) in the periodic table.
1) The atomic mass of Lithium is 6.94 u.
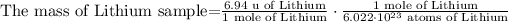
2) We can cancel some units and rearrange the equation.
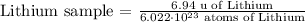
3) Multiply the equation above by the number of atoms
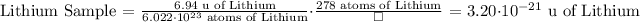
The mass of 278 atoms of Lithium is 3.20*10^-21 u