Answer
89.0 g
Step-by-step explanation
Given information:
Equation of the reaction: 2Na + Cl₂ → 2NaCl₂
Reacting mass of Na = 35.0 g
Reacting mass of Cl₂ = 100.0 g
What to find:
The maximum amount of NaCl that can be produce from 35.0g of Na and 100.0g of Cl₂.
Step-by-step solution:
From the given balanced chemical equation for the reaction; 2 moles of Na reacts with 1 mole of Cl₂ to produce 2 moles of NaCl₂.
The next step is to convert the given masses to moles
From the Periodic Table:
Molar mass of Na = 22.989769 g/mol
Molar mass of Cl₂ = 35.453 g/mol
The formula to calculate mole is:
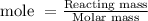
Therefore,
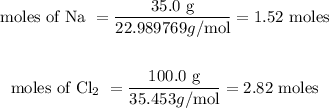
The next step is to determine the limiting reactant.
To get that, divide the moles of Na and Cl₂ with their respective coefficients in the given equation:
For Na = 1.52/2 = 0.75
For Cl₂ = 2.82/1 = 2.82
Hence, Na is the limiting reactant.
Now you can compare the mass of the mass of Na to the mass of NaCl₂ produced as follows:

The maximum amount of NaCl that can be produce from 35.0g of Na and 100.0g of Cl₂ is 89.0 g