Answer:
Adding a piece of Zn
Step-by-step explanation:
Adding zinc (Zn) to a hydrochloric (HCl) solution will prove its acidity with a redox reaction:
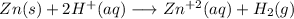
This reaction can be confirmed, by the observation of little bubbles in the surface of the metal: that bubbles are the hydrogen gas generated.