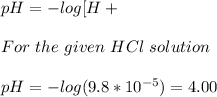Answer:
pH of the given HCl solution is 4.0
Step-by-step explanation:
Given:
Concentration of HCl solution = 9.8*10⁻⁵ M
To determine:
The pH of the solution
Step-by-step explanation:
pH of a solution refers to the concentration of H+ ions which in turn reflects the acidity of the solution
pH is related to the H+ concentration as follows: