We are asked to prepare a solution starting from a stock solution with a concentration equal to 1.0M. This is the initial concentration C1. We must find the volume that we must take from this solution to obtain a final solution with a final concentration equal to 0.25M and a volume equal to 100mL.
We will apply the following equation:
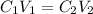
Where,
C1 is the initial concentration of the solution, 1.0M
V1 is the initial volume of the solution, unknown
C2 is the final concentration of the solution, 0.25M
V2 is the final concentration of the solution, 100mL
We clear V1 and replace the known data:
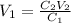
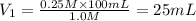
Answer: To prepare the requested solution we must take 25mL of the stock solution and complete it with distilled water up to 100mL. The solution will have 25 mL of stock solution and 75 mL of distilled water.