Answer
282.47 g
Step-by-step explanation
Given:
Molality of solution = 1.2 M
Kilogram of water = 2.40 kg
What to find:
Mass of H₂SO₄
Step-by-step solution:
Molality of solution is given by:
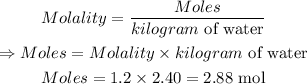
The next is to convert 2.88 mol H₂SO₄ to mass.
1 mol H₂SO₄ = 98.079 g
2.88 mol H₂SO₄ = 2.88 x 98.079 = 282.47 g
The mass of H₂SO₄ (sulfuric acid) must be dissolved into 2.40 kg h2o to produce a 1.20 m solution is 282.47 g