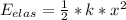Answer:
See the explanation below.
Step-by-step explanation:
Energy is the ability of bodies to perform work and produce changes in themselves or other bodies.
There are several types of energy, but let's talk specifically about mechanical energy.
Mechanical energy is associated or subdivided into kinetic, potential, and elastic energies.
Kinetic energy
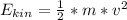
Potential energy
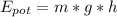
Elastic Energy