Answer:
Oxygen is the limiting reactant for this reaction
Step-by-step explanation:
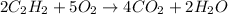
According to reaction, 2 moles of acetylene reacts with 5 moles of oxygen.
If there 37.0 moles of acetylene, then they will react with:
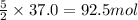
If there 81.0 moles of oxygen, then they will react with:
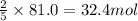
As we can see that 81 moles of oxygen will completely react with 32.4 moles of acetylene.
But we are given with 37 moles of acetylene which means that oxygen is present in limiting amount and acetylene is present ion excessive amount.