Answer:
B; Some of the products formed from the experiment escaped to the surroundings as gas.
Step-by-step explanation:
We are given that Brie is Studying the law of conservation of mass at school , so she decides to test it.First , she measures and records the mass of 1 teaspoon of baking soda and 1 table spoon of vinegar .
We have to find best explains the mass of reactants in Brie's experiment was greater than the mass of products.
Then , she combines the two substances and observes
The chemical formula of baking soda is
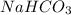 (Base)
(Base)
The chemical formula of vinegar is
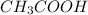 (Acid)
(Acid)
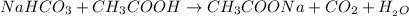
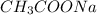 = Sodium Acetate (Salt)
= Sodium Acetate (Salt)
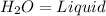 =Water
=Water
 =Carbon dioxide=Gas
=Carbon dioxide=Gas
When we combine 1 teaspoon sodium bicarbonate and 1 teaspoon vinegar
Then sodium acetate salt , water and gas carbon dioxide are formed.
she. notices that mixture bubbles of carbon dioxide. vigorously .After the mixture stops bubbling, she measures and records the mass of it .
When she examine her data , she notices that the mass of mixture is less than the mass of the vinegar and baking soda added together because carbon dioxide is a gas which escape into surrounding . Therefore, the mass of product decreases due to escaping of gas.
Brie, is confused because it appears as if mass not conserved.
The mass of reactants is greater than the mass of products due to escaping of gas into surrounding. So , the mass is not conserved in Brie's experiment.
Answer:B; Some of the products formed from the experiment escaped to the surroundings as gas.