Answer:
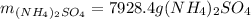
Step-by-step explanation:
Hello,
In this case, the undergoing chemical reaction is:
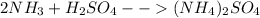
Now, by taking into account that 60.0 mol of sulfuric acid are used, the produced grams of ammonium sulfate, by applying the stoichiometric factors, turn out as shown below:
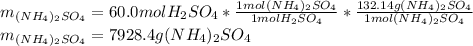
Best regards.