Answer:
The partial pressures of O₂ and CO₂ are 0.489 atm and 0.511 atm respectively.
Step-by-step explanation:
From the Questions we are given;
Volume = 10 Liter
Temperature = 298 K
Pressure = 1 atm
We need to calculate the partial pressures of O₂ and CO₂
Step 1 : Number of moles of gaseous mixture
Using the ideal gas equation;
PV =nRT, where P is the pressure, V is the volume, n is the number of moles, T is the temperature in K and R is the ideal gas constant (0.082 L∙atm/K∙mol)
Therefore;
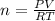
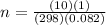
Solving for n
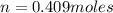
Step 2: Moles of CO₂
Total number of moles of the mixture = 0.409 moles
Moles of Oxygen = 0.2 moles
Therefore;
Moles of CO₂ = 0.409 moles - 0.2 moles
= 0.209 moles
Step 3: Partial pressures of O₂ and CO₂
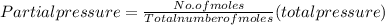
Therefore;
Partial pressure of Oxygen gas
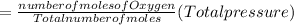
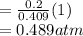
Partial pressure of CO₂
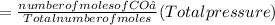
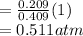
Thus, the partial pressures of O₂ and CO₂ are 0.489 atm and 0.511 atm respectively.