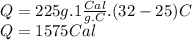Answer:
1575 Cal
Step-by-step explanation:
Between this temperature range, the water does not change state since its boiling point is at 100 degrees Celsius.
As there is no change of state we use the sensible heat formula
The specific heat of liquid water is
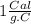
We substitute in the formula