They give us the concentration of the solution in terms of molarity. Molarity is defined as the moles of solute per liter of solution, that is, it has the following equation:
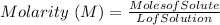
They give us the molarity and the volume, so the mol of solute ( will be:
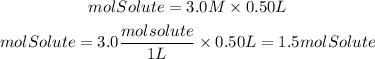
The solute is iron(I) nitrate, Fe(NO3)2. To calculate the grams of Fe(NO3)2 we will multiply the moles of Fe(NO3)2 by its molar mass, 179.86g/mol.
So, the grams of Fe(NO3)2 will be:
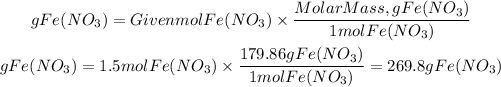
There are 269.8g of Fe(NO3)2. So, the answer will be A)269.81 g