Answer:
Option (b) is correct.
 of
of
 values describes the most acidic solution
values describes the most acidic solution
Explanation:
Given : Some
 values .
values .
We have to determine which of the following
 values describes the most acidic solution.
values describes the most acidic solution.
Since, pH defines the acidity of a solutions and shows the presence of
 in it . Its range varies from 1 to 14 and with increase in number the acidity of the solution decreases and this range of 1 to 14 defines the negative power of 10 in the solution.
in it . Its range varies from 1 to 14 and with increase in number the acidity of the solution decreases and this range of 1 to 14 defines the negative power of 10 in the solution.
Also, as the concentration of H+ increases the acidic nature of solution increases.
Thus, For solution to be acidic the range is from 1 to 6, thus from the given options
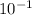 defines the most acidic solution.
defines the most acidic solution.
Thus,
 of
of
 values describes the most acidic solution
values describes the most acidic solution