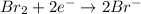Answer : The correct option is,
Explanation :
Electrolytic cell : It is defined as the cell in which the chemical reaction occurs by passing of the current from external source.
In this electrolytic cell, the oxidation occurs at anode which is a positive electrode and reduction occurs at cathode which is a negative electrode.
For example : On passing electricity in the molten
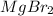 .
.
The two half-reaction equation are :
Reduction :
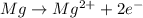 (cathode)
(cathode)
Oxidation :
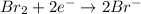 (anode)
(anode)
The balanced chemical reaction will be,
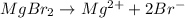
Hence, the correct option is,