Let's determine what will happen to the volume of an ideal gas if the temperature is reduced while the pressure is kept constant.
Apply the ideal gas law:

Where:
P is the pressure
V is the volume
n is the number of moles
R is the universal gas constant
T is the temperature.
Here, we have:
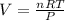
When the temperature decreases and pressure is kept constant, the volume of the of the ideal gas will also decrease since the volume of directly proportional to the temperature and inversely proportional to the pressure.
ANSWER:
B). Decrease