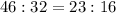Answer
The mass ratio of Na to S in Na₂S is 23 : 16
Step-by-step explanation
The molecular formula of sodium sulfide is Na₂S
From the periodic table,
Molar mass of Na = 23 g
Molar mass of S = 32 g
The total mass of Na in Na₂S = 2 x 23 g = 46 g
The total mass of S in Na₂S = 32 g
Therefore the mass ratio of Na to S in Na₂S is