Answer : The more moles of carbon there than sulfur are, 0.52 moles.
Explanation : Given,
Mass of carbon = 10 g
Mass of sulfur = 10 g
Molar mass of carbon = 12 g/mol
Molar mass of sulfur = 32 g/mol
First we have to calculate the moles of carbon and sulfur.
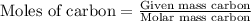
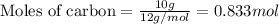
and,
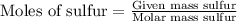
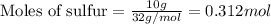
From this we conclude that,
Moles of carbon = 0.833 mol
Moles of sulfur = 0.312 mol
Difference between the moles of carbon and sulfur = 0.833 - 0.312 = 0.521 mol = 0.52 mol
Thus, the more moles of carbon there than sulfur are, 0.52 moles.