ANSWER
250 kJ
Step-by-step explanation
The given amount of lead is at its melting point. Every change of phase is produced at a constant temperature, so to find how much heat is needed to melt this lead, we only need the latent heat of fusion,
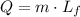
The mass m = 10kg and Lf = 0.25x10⁵ J/kg,
![Q=10\operatorname{kg}\cdot0.25*10^5J/kg=250,000J=250kJ]()
Hence, the amount of heat needed to melt 10kg of lead at its melting point is 250 kJ