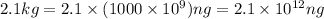Answer: The mass of the sample having 2.1 kg of
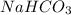 will be
will be
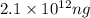
Explanation: We are given a sample of sodium bicarbonate
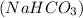 having mass 2.1 kg
having mass 2.1 kg
To convert this into nano-grams, we use the following conversions:
1 kg = 1000 grams
and
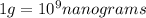
Converting 2.1 kg to nano-grams using above conversions: