In this problem, we have to use the Boyle's law. But first, let's remember this concept:
Boyle's law states that the volume of a given mass of gas varies inversely with the pressure when the temperature is kept constant. An inverse relationship is described in this way. As one variable increases in value, the other variable decreases.
So, what is happening? An increase in pressure pushes the molecules closer together, reducing the volume. If the pressure is decreased, the gases are free to move about in a larger volume.
The formula of Boyle's law is:
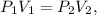
where P is pressure in atm and volume is in liters (L).
Now, we need to know what is the value of pressure in atm. Remember that 1 atm is the same that 760 torr. The calculation is:
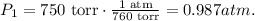
So, our initial data is:
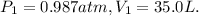
And the final data is:
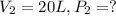
The problem is asking for the final pressure which is P2. To obtain this pressure, we need to clear P2 in the Boyle's law formula, like this:
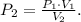
And then, we have to replace the data that we have on the formula:
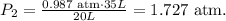
Pressure is in atm, so let's do the conversion to torr:
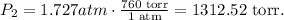
The answer is that the final pressure is 1.727 atm which is 1312.52 torr too. The volume is decreasing, so the pressure is increasing.