Answer:
A gas that exerts a pressure of 583.33 psi. psi in a container with a volume of 12.45 L will exert a pressure of 1112 psi when transferred to a container with a volume of 6.531 L.
Step-by-step explanation:
Initial pressure exerted by the gas =
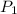
Initial volume occupied by the gas =
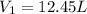
Final pressure exerted by the gas =
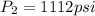
Final volume of the gas occupied by the gas =
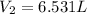
Applying Boyle's law:
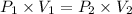
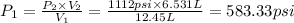
The initial pressure exerted by the gas was 583.33 psi.
A gas that exerts a pressure of 583.33 psi. psi in a container with a volume of 12.45 L will exert a pressure of 1112 psi when transferred to a container with a volume of 6.531 L.