Given:
A 76,900-liter swimming pool has a chlorine concentration of 2.6 ppm.
To find:
How many liters must be replaced with 20 ppm chlorine solution to increase the chlorine concentration to 4 ppm?
Solution:
The concentration in terms of ppm is given by:
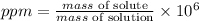
So, the mass of solute becomes:
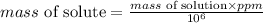
Let x mL of the pool solution is replaced by 20 ppm chlorine solution to make final chlorine concentration equal to 4 ppm. So,
![\text{ mass of the solute added}+\text{ mass of the solute unchanged}=new\text{ mass of the solute in the solution}]()

Thus, the answer is 6117.1 L.