The mass number of an atom represents the number of protons and neutrons.
For the given case, the mass number is 20.
This means that the sum of the number of protons and neutrons is 20.
It is also given that the number of neutrons is 4.
So, the number of protons is
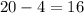
We know that there is an equal number of protons and electrons in a neutral atom.
This means that there must be 8 protons and 8 electrons in the atom.
Therefore, there are 8 electrons in the last shell of the atom.