Given:
Work done, W = 5 J
Initial energy = 8J
Final energy = 30J
Let's determine if the work done have a positive or nrgative value.
Appy the equation for the first lae of thermodynamics:
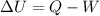
Where:
U is the change in internal energy
Q is the added heat
W is the work done
To find the work done here, we have:
Rewrite the formula for W
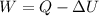
Where:
ΔU = 30J - 8J = 22J
Q = 5J
Thus, we have:
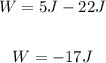
Therefore, the work done here is -17J.
This means the work done in this scenario has a negative value.
ANSWER:
The work done in this scenario has a negative value