Answer:
500 g of NaCl.
Step-by-step explanation:
The given data is the volume (0.5 L) and density (1 g/mL). The question is asking for mass (grams). From volume and density, we can find the mass using the following formula:
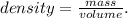
And solving for mass, we're going to have:
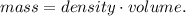
But we have a problem. We need to use the correct units to find the mass. We can convert 0.5 L to mL (milliliters). Remember that 1 L equals 1000 mL, so the conversion is:
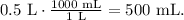
And now, with this volume, we can find the mass using the formula of mass:
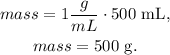
We're going to need 500 g of NaCl to make 0.5 L of a 1 g/mL solution.