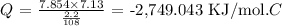Answer:
Step-by-step explanation:
Here, we want to calculate the heat of combustion per gram of quinone
By principle of thermodynamics,
The heat lost by burning the quinone = heat gained by the calorimeter
The temperature rise is:
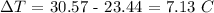
The heat of combustion per gram can be calculated as:
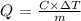
where C is the calorimeter heat capacity and m is the mass of the quinone
Thus,we have this as:
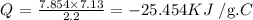
The heat is negative as it is exothermic since it is given off
To get the heat of combustion per mole, we have to multiply the heat of combustion per gram by the molar mass of quinone
The molar mass of quinone is 108 g/mol
So, we have the heat of combustion per mole calculated as: