ANSWER
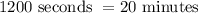
Step-by-step explanation
To calculate the half-life of the sample, apply the formula for the quantity remaining in a radioactive decay:
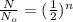
where N/No = ratio of quantity remaining to initial quantity = 1/16
and n is:
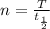
where T = time elapsed = 80 mins = 4800 seconds
t1/2 = half-life
Solving for n in the equation above:
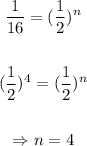
Therefore, the half-life of the sample is:
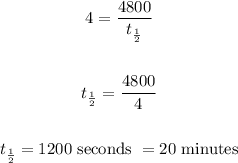
That is the answer.