ANSWER
The mass of NaOH that reacted is 219.58 grams
Step-by-step explanation
Given that
The mass of Fe(OH)3 is 196 grams
Follow the steps below to find the mass of NaOH
Step 1; Write the balanced equation of the reaction
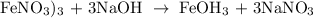
Step 2; Find the moles of Fe(OH)3 using the below formula
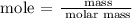
Recall, that the molar mass of Fe(OH)3 is 106.87 g/mol
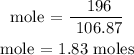
The number of moles of Fe(OH)3 is 1.83 moles
Step 3; Find the number of moles of NaOH using a stoichiometry ratio
In the equation above, 1 mole of Fe(OH)3 gives 3 moles of NaOH
Let x represents the number of moles of Fe(OH)3
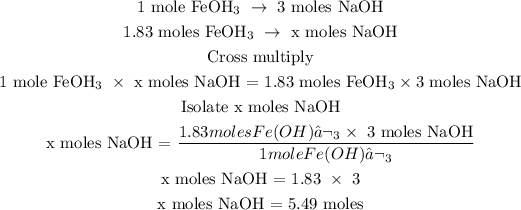
The number of moles of NaOH is 5.49 moles
Step 4; Find the mass of NaOH using the below formula
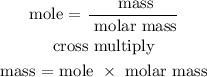
Recall, that the molar mass of NaOH is 39.997 g/mol
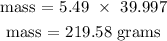
Therefore, the mass of NaOH that reacted is 219.58 grams