Answer
114.841 grams
Step-by-step explanation
Given:
Moles of C = 4.1 mol
What to find:
Mass in grams of CO in 4.1 moles of CO.
Solution:
The first steF is to find the molar mass of CO.
rom the periodic table, the atomic masses of (C = 12.011, O = 15.999)
Molar mass of CO = 12.011 + 15.999 = 28.01 g/mol
The final step is to calculate the mass of CO in grams using the mole formula
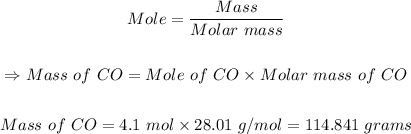
114.841 grams of CO are in a 4.1 mole sample of CO