The reaction of 64.23 g of
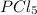 with excess water produces 22.51 g of
with excess water produces 22.51 g of
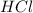
In scientific notation:
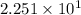 g HCl
g HCl
To determine the mass of hydrogen chloride produced when 64.23 g of phosphorus pentachloride
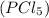 reacts with excess water, we can use stoichiometry and the balanced chemical equation:
reacts with excess water, we can use stoichiometry and the balanced chemical equation:
![\[ PCl_5 (s) + H_2O(l) \rightarrow POCl_3 (l) + 2HCl(aq) \]](https://img.qammunity.org/qa-images/2023/formulas/chemistry/college/jou604kryr4it11vry0y.png)
First, find the molar mass of
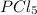 and
and
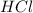 :
:
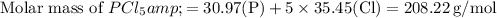
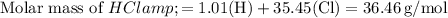
Next, determine the moles of
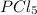 using its given mass:
using its given mass:
![\[\text{Moles of } PCl_5 = \frac{\text{Mass}}{\text{Molar mass}} = \frac{64.23 \, \text{g}}{208.22 \, \text{g/mol}} = 0.3087 \, \text{mol}\]](https://img.qammunity.org/qa-images/2023/formulas/chemistry/college/nyx7mnezrynmdztkdblp.png)
According to the balanced equation, 1 mole of
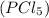 produces 2 moles of
produces 2 moles of
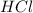 . Therefore, moles of
. Therefore, moles of
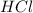 produced:
produced:
![\[\text{Moles of } HCl = 2 * \text{Moles of } PCl_5 = 2 * 0.3087 \, \text{mol} = 0.6174 \, \text{mol}\]](https://img.qammunity.org/qa-images/2023/formulas/chemistry/college/j7fgs03nnaz5mcc03ohd.png)
Finally, convert moles of
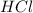 to grams:
to grams:
![\[\text{Mass of } HCl = \text{Moles} * \text{Molar mass} = 0.6174 \, \text{mol} * 36.46 \, \text{g/mol} = 22.51 \, \text{g}\]](https://img.qammunity.org/qa-images/2023/formulas/chemistry/college/z8z3l8ccy3ncl82032ax.png)
So, when 64.23 g of
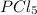 reacts with excess water, 22.51 g of
reacts with excess water, 22.51 g of
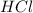 will be produced.
will be produced.