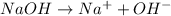Answer: A base gives off hydroxide ions.
Explanation: According to Arrhenius concept, a base is defined as a substance which donates hydroxide ions
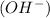 when dissolved in water and an acid is defined as a substance which donates hydronium ions
when dissolved in water and an acid is defined as a substance which donates hydronium ions
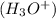 in water.
in water.
According to the Bronsted Lowry conjugate acid-base theory, an acid is defined as a substance which donates protons and a base is defined as a substance which accepts protons.
Acids have pH values ranging from 1 to 6.9, neutral solutions have pH of 7 and bases have pH ranging from 7.1 to 14.
A substance that increases the hydroxide ion concentration of a solution is an Arrhenius base.