Answer: Hence, 2 moles of NaOH would neutralize 1 mole of

Step-by-step explanation:

One mole of hydrochloric acid neutralizes one mole of sodium hydroxide to give one mole of sodium chloride and one mole of water
When sulfuric acid is used in the place of HCl , 1 mole of sulfuric acid will neutralizes the 2 mole of sodium hydroxide and gives one mole of sodium sulfate and 2 moles of water. As we can see from the reaction:
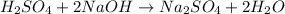
Hence, 2 moles of NaOH would neutralize 1 mole of
