Answer:
142.0 grams
Step-by-step explanation:
We were given the following details:
trace element concentration, c = 0.044 grams per cubic centimeter of water
Charlotte uses a container in the shape of a right cylinder with:
Radius, r = 9.8 cm
Height, h = 10.7 cm
The sample contains the same proportion of the trace element. The amount of trace element collected is calculated as shown below:
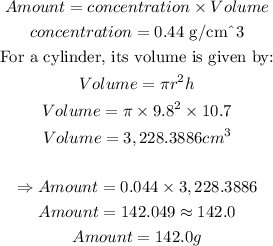
Therefore, the amount of trace element is 142.0 g