Answer
-52.2 Joules
Step-by-step explanation
Given that;
Mass of krypton, m = 12.3 g
Temperature change, ΔT = 22.2°C - 39.3°C = -17.1°C
The specific heat of gaseous krypton, c = 0.248 J/g°C.
What to find:
The energy change, Q.
Step-by-step solution:
The energy change, Q can be determined using:
Q = mcΔT
Putting the values of the given parameters into the formula, this yields:
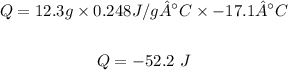
Therefore the energy change = -52.2 Joules