A.1.2°C
Step-by-step explanationThe First Law of Thermodynamics states that heat is a form of energy, and thermodynamic processes are therefore subject to the principle of conservation of energy.
To find internal energy, you have to add the heat added in the system and work done in the system because the work done is not lost but rather it is added in the system.
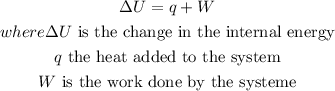
Step 1
a)let
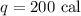
to add the energy it must have the same measure unit, so let's convert calories into Julies
remember that
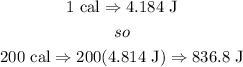
b) now, replace in the formula
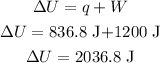
now, we have the change of internal energy
Step 2
now, let's find the change in temperature
Use the calorimetry formula.
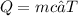
where m is the mass, Q = heat energy, c = specific heat capacity, and ∆T = change in temperature
a)
let
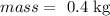
now,

so, the answer is
A.1.2°C