So,
First of all, remember that a chemical equation has the following general form:
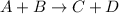
Compound A reacts with compound B, to form compounds C and D.
In this question, we have that 5.92 g of sodium oxalate is reacted with 5.92 of calcium chloride. So, we can identify the following:
Balanced chemical reaction:
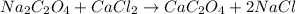
This reaction is a displacement reaction.
The reaction tells us that sodium oxalate is reacted with calcium chloride to form calcium oxalate and sodium chloride.
Formula for reactant A: (Sodium oxalate)
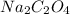
$$Na_2C_2O_4$$Grams of reactant A: 5.92g
Formula of reactant B: (Calcium chloride)
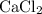
Grams of reactant B: 5.92g
Formula of product C: (Calcium oxalate)
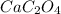
Formula of product D: (Sodium chloride)
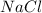
To find the amount of grams of calcium oxalate produced (Product C), we could use the stoichiometry of the reaction. But first, we need to pass all the amounts of each reactant to moles (n). (This is the first step)
This is, just divide the amounts by the molar mass of each compound:
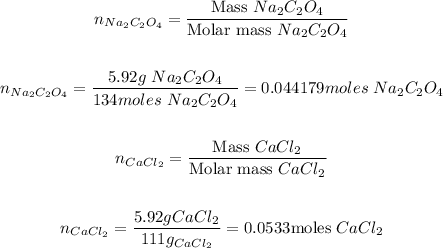
Now, let's apply the stoichiometry of the reaction using the limiting reactant. (In this reaction, the limiting reactant is the sodium oxalate).
In the reaction, we can clearly see that for each mol of sodium oxalate, two 1 mol of calcium oxalate is produced. So:
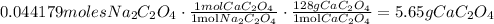
So, 5.65 grams of Calcium oxalate are produced.
We use the same process to find the grams of product D. But, notice that in the reaction, for each mol of sodium oxalate, two moles of NaCl reacts. Then,
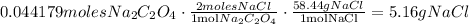
So, 5.16 g of NaCl are produced.