Answer : The mole ratio for aluminium chloride to chlorine is, 2 : 3
Explanation :
The given unbalanced chemical reaction is :
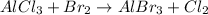
First we have to balance the chemical reaction.
In order to balance the chemical reaction, the coefficient 2 is put before
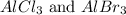 and the coefficient 3 is put before
and the coefficient 3 is put before
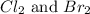 .
.
The given balanced chemical reaction will be :
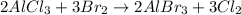
By the stoichiometry we can say that, 2 moles of
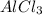 react with 3 moles of
react with 3 moles of
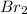 to give 2 moles of
to give 2 moles of
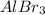 and 3 moles of
and 3 moles of
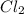 as a product.
as a product.
That means,
The mole ratio between the
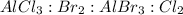 are
are
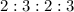 respectively.
respectively.
Thus, the mole ratio for aluminium chloride to chlorine is, 2 : 3