Here, the volume is constant as the metal container is sealed.
The temperature increased by a factor of 4.78x.
According to ideal gas law,
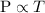
Here, P is the pressure and T is the temperature.
As the temperature increases by 4.78x, so the pressure will increase by 4.78x.