In this case, all the values, the one we have and the one we want we to calculate, are in moles, so we won't need to convert from or to mass.
If all 25 moles of S₈ react, the amount that will be produced of H₂S will follow the coefficients of the reaction.
The coefficient of S₈ is 1 (it is implicit) and the coefficient of H₂S is 8.
So, we write columns for each, the first column we put S₈ and its coefficient, and the second we put H₂S and its coefficient:
S₈ --- H₂S
1 --- 8
Now, this is similar to a rule of three, so we will have a fraction of the number of moles of S₈ over 1 in one side and a fraction of the number of moles of H₂S over 8 on the other side:
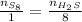
Now, we just need to solve for n of H₂S:
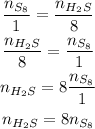
Since we have 25 moles of S₈, we have:
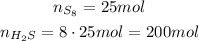
So, we will produce 200 moles of H₂S.