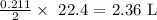Answer:
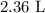
Step-by-step explanation:
Here, we want to know the volume of chlorine gas necessary to complete the reaction
We can start by writing a balanced equation of reaction as follows:
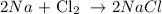
From the balanced equation, we can see that 1 mole of chlorine gas requires 2 moles of sodium metal
Now, let us get the number of moles of sodium metal that actually reacted
We have that as the mass of sodium metal divided by the atomic mass
The atomic mass of sodium metal is 23 amu
Thus, we have the number of moles as:
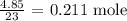
Since the mole ratio is 2 to 1, half of this number of moles is needed by the chlorine gas
Mathematically, 1 mole of a gas occupies a volume of 22.4 L at STP
the volume occupied by the calculated number of moles will be: