When an object loses an electron, it gains more charge and becomes more positively charged. So, if an object originally has a charge of -3.5 x 10⁻⁶ C, it must gain a charge of [ 1.4 x 10⁻6 - (-3.5 x 10⁻⁶) } = 4.9 x 10⁻⁶ C. This results to a final charge of 1.4 x 10⁻6 C.
Recall that one electron has a charge of 1.6 x 10⁻¹⁹ C. This means that for an object to gain a charge of 4.9 x 10⁻⁶ C, the number of electrons that an object must lose is
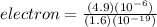
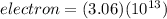
Thus, the object must remove a total of 3.06 x 10¹³ electrons.
Answers: 3.06 x 10¹³ electrons