ANSWER
The heat of reaction of the above reaction is -234 KJ
Step-by-step explanation
Given information
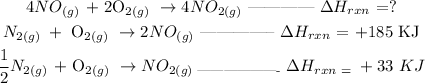
Recall, that change in enthalpy of the reaction is the summation of the products - summation of the reactants
Mathematically,
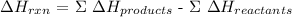
The next step is to write the formula for calculating the enthalpy
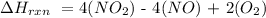
The next step is to substitute the given data into the above formula
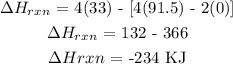
Hence, the heat of reaction of the above reaction is -234 KJ